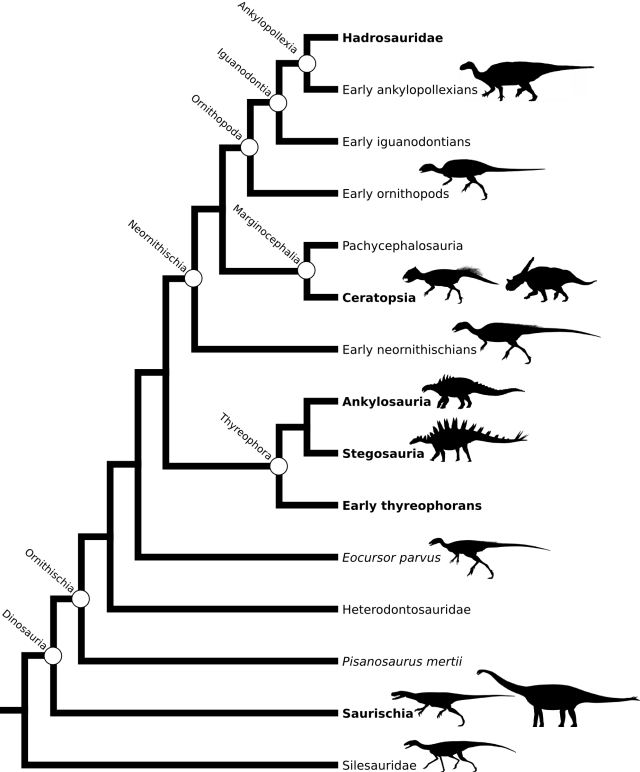
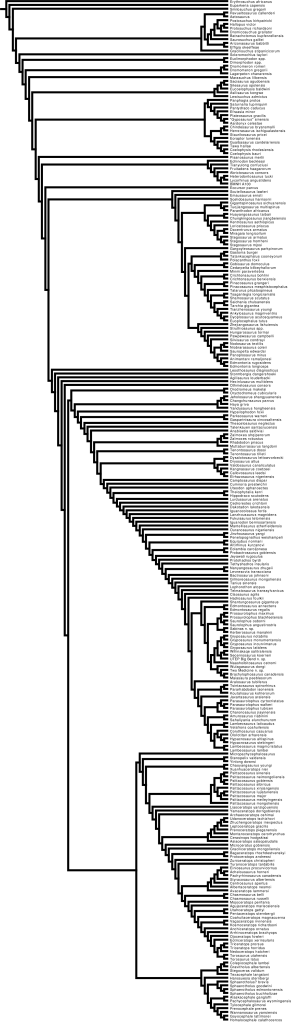
As mentioned in the previous post, a few papers with new or updated phylogenies for various ornithischians have appeared in the last year. Thus, I spent a few hours going through them and updating the tree topology as well as adding taxa. The phylogeny is given below, with notes on what I did at the end of the post. Note that many of the arbitrarily-resolved nodes aren’t that critical for some aspects of the analysis; they often include fragmentary taxa (sometimes only known from cranial material). We may wish to revise some of the resolutions in order to reduce ghost lineages.
I have not yet incorporated Sterling Nesbitt’s most recent phylogeny of Archosauria, because I wasn’t able to successfully download the file. It’s top of my list once I do.
If you have suggestions to revise or improve the phylogeny, please mention them in the comments.

ODP Phylogeny Draft, 24 June 2011
Notes
General Ornithischia
General phylogeny of Ornithischia updated following 50 percent majority-rule consensus tree in Butler et al. 2011, as modified by Makovicky et al. 2011 (the latter with modifications of codings for Thescelosaurus and addition of Haya)
Position of Thescelosaurus updated following Makovicky et al. 2011, modified from Butler et al. 2011
Position of Talenkauen follows Butler et al. 2011
Updated the position of Yinlong, Micropachycephalosaurus, Stenopelix. Sister taxon relationship of Yinlong+Stenopelix follows Butler et al. 2011; relationships of Micropachycephalosaurus+Chaoyangsaurus+(Stenopelix+Yinlong)+(rest of ceratopsians) resolved arbitrarily.
Topology within Heterodontosauridae after Pol et al. 2011 and after Butler et al. 2011. Echinodon was placed as a basal heterodontosaurid heterodontosaurid (following Butler et al. 2011). The clade of Abrictosaurus+NHM A100+Heterodontosaurus+Lycorhinus was resolved arbitrarily.
Bugenasaurus and Thescelosaurus synonymized following recent work by Boyd et al. (2009).
Ceratopsia
Topology of Chasmosaurinae follows Sampson et al. 2010; placement of Ojoceratops and Eotriceratops resolved arbitrarily. Triceratops, Diceratops, and Torosaurus spp. are considered separate, following Farke 2011.
Topology of Ceratopsia follows Makovicky 2010; inclusion of Micropachycephalosaurus and Stenopelix follows Butler et al. 2011. Placement of Zhuchengceratops inexpectus follows Xu et al. 2010, with Zhuchengceratops and Udanoceratops arbitrarily placed as sister taxa (based on biogeography).
Topology of Psittacosauridae follows Sereno 2010, figure 2.23E. P. gobiensis is placed arbitrarily as sister to P. sibiricus. Psittacosaurus ordosensis is synonymized with P. sinensis, following a tentative suggestion from this paper. Psittacosaurus xinjiangensis is placed following the polytomy in Averianov et al. 2006, with its resolution arbitrary.
Pachycephalosauria
Topology within Pachycephalosauria follows Longrich et al. 2010. In recognition of the probably synonymy of Pachycephalosaurus, Stygimoloch, and Dracorex, the latter two animals were removed from the matrix and the matrix was rerun in PAUP otherwise following the specifications of Longrich et al. 60 equally parsimonious trees resulted; in order to more completely resolve relationships within the clade, the 50% majority rule tree was used for the topology within Pachycephalosauria. ?Sphaerotholus brevis was arbitrarily placed at the base of the clade including all other Sphaerotholus species, and Texacephale was arbitrarily placed at the base of the clade including Stegoceras+Gravitholus+Colepiocephale.
Ankylosauria
Topology within Ankylosauria updated following Burns et al. 2011, Figure 8 (50% majority rule consensus tree).
The placement of Cedarpelta as an ankylosaurid follows Lü et al. 2007 and an unpublished analysis by Nick Gardner (http://whyihatetheropods.blogspot.com/2008/12/cedarpelta-as-ankylosaurid.html). Ultimately, no postcrania for this taxon are included, so its placement is not terribly critical. Cedarpelta and Gobisaurus placed as sister taxa following this same analysis.
Dyoplosaurus is recognized as a unique taxon (Arbour 2009), and is arbitrarily placed as sister to Euoplocephalus.
Aletopelta is tentatively given as an ankylosaurid, based on text in Ford and Kirkland 2001.
The polytomy between Tianzhenosaurus, Nodocephalosaurus, and Ankylosaurus was resolved arbitrarily.
Niobrarasaurus and Nodosaurus are arbitrarily given as sister taxa, because of their general similarity. Furthermore, Coombs (1990), in The Dinosauria, suggested that Nodosaurus is close to Sauropelta and Silvisaurus (p. 478, caption for Figure 22.14, “Nodosaurus probably fits just above or just below node 8.” [i.e., either between Sauropelta and Silvisaurus, or Silvisaurus and Panoplosaurus], so this opinion is followed here. Based on geography alone, they are given as sister to Sauropelta.
Placement of Crichtonsaurus was semi-arbitrary; the tree of Lu et al. with this taxon cannot be easily reconciled with the Burns et al. phylogeny. Noting that both phylogenies recover Pinacosaurus “above” Gobisaurus; Crichtonosaurus was placed between the two. Crichtonsaurus‘s placement “above” Minmi was arbitrary, based on the later geological occurrence of Crichtonsaurus.
Polacanthus foxii is arbitrarily given the basal position within Nodosauridae occupied by Hylaeosaurus, for Coombs 1990, Fig. 22.14.
Zhejiangosaurus is completely arbitrarily placed as more derived than Polacanthus.
Because of great uncertainty in phylogenetic position, and because no phylogenetic analysis has adequately addressed the taxa, I recommend removing Aletopelta, Niobrarasaurus, Nodosaurus, Polacanthus, and Zhejiangosaurus from the analysis.
The topology of nodosaurids (including the placement of Hungarosaurus and Struthiosaurus) follows Ösi 2005, Figure 15. The monophyly of Edmontonia follows Burns et al. 2011.
Basal iguanodonts
Topology of basal iguanodonts follows McDonald 2011, Figures 1 and 2.
Genus of “Camptosaurus” aphanoecetes changed to Uteodon, per McDonald 2011
Muttaburrasaurus placed in Rhabdodontidae following McDonald et al. 2010. Its resolution at the base of the clade is arbitrary, but based on the fact that it occurs much earlier in the fossil record than other rhabdodontids.
Monophyly of Zallovosaurus+Dryosaurus+Dysalotosaurus+Elrhazosaurus+Kangnasaurus+Valdosaurus follows McDonald et al. 2010. Resolution within that clade is arbitrary. Dysalotosaurus is made its own genus, following recent work.
“Dollodon bampingi” and M. atherfieldensis are synonymized following McDonald 2010.
Resolution of Cumnoria and Uteodon is arbitrary relative to their polytomy.
Resolution of Cedrorestes, Dakotadon, Lanzhousaurus, and Iguanacolossus relative to their polytomy is arbitrary. Lanzhousaurus as being part of less inclusive clade follows Figure 2 of McDonald.
Position of Tethyshadros, Nanyangosaurus, and Tanius adjusted in light of McDonald figures 1 and 2. Position of Telmatosaurus as part of clade excluding Lophorothon also follows this analysis.
Levnesovia and Nanyangosaurus arbitrarily made sister taxa; also made part of clade excluding Tethyshadros, following Figure 2 of McDonald. Probactrosaurus and Eolambia were arbitrarily made sister taxa to resolve a polytomy.
Altirhinus and Equijubus were arbitrarily made sister taxa, and arbitrarily placed as part of clade excluding Jinzhousaurus+Penelopognathus in order to resolve polytomy.
Iguanodon placed outside of more derived iguanodonts+Mantellisaurus. Position of Ouranosaurus is arbitrarily resolved.
Lambeosaurinae
Topology of Lambeosaurinae modified following Evans 2010 (Hypacrosaurus paper), Figure 16A (parsimony trees, strict consensus).. Pararhabdodon+Koutalisaurus+Tsintaosaurus retained from “old” Prieto-Marquez phylogeny, because the first two taxa are not on the Evans phylogeny. Nipponosaurus was deleted, because it is an obvious juvenile and many characters cannot be scored effectively. Amurosaurus+Sahaliyania retained from previous version of phylogeny, because latter taxon is not on Evans phylogeny. Polytomy of Corythosaurus and Olorotitan resolved following Bayesian tree (Fig. 16B). Lambeosaurus laticaudatus and Velafrons are arbitrarily resolved. Velafrons placed as closer to corythosaurins follows the original Gates et al. 2007 description of the taxon (Velafrons is not in the Evans phylogeny).